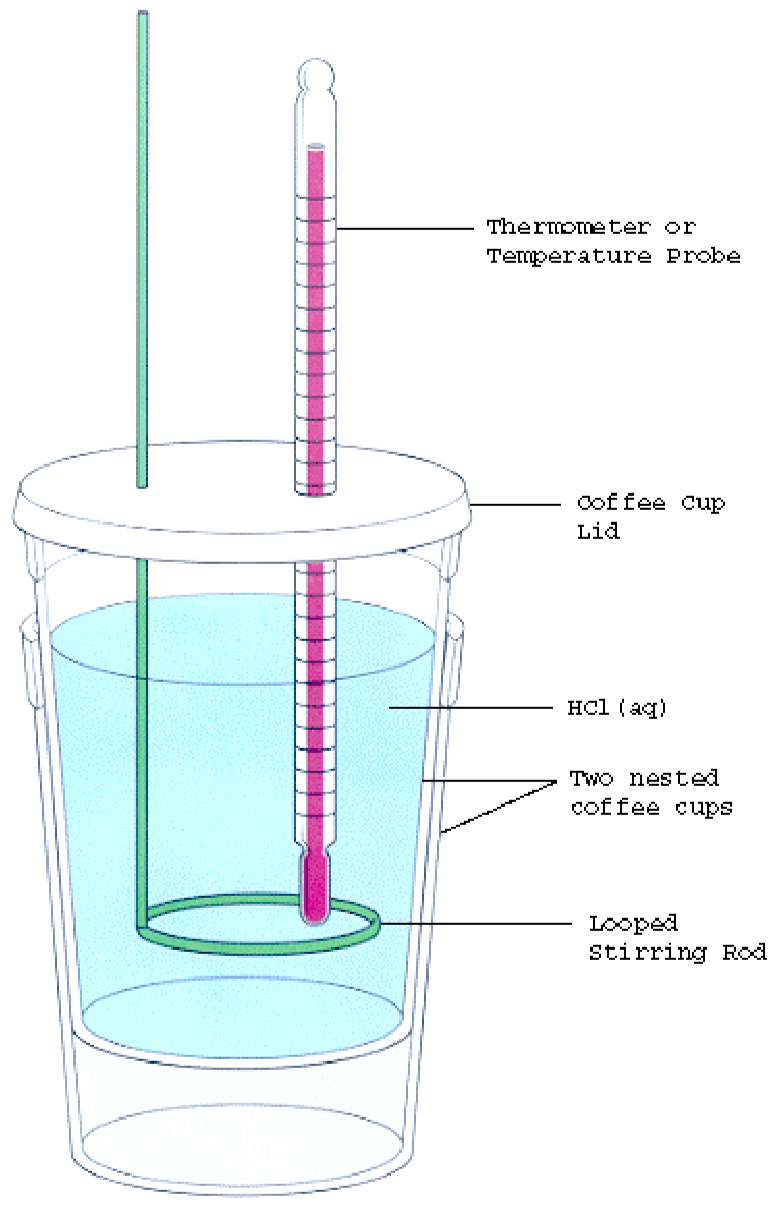
Purpose Of Calorimeter Lab . a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. A doubled styrofoam cup fitted. To remind you, endothermic reactions gain energy from the. calorimetry is the science of measuring heat flow. Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from chem.libretexts.org
A doubled styrofoam cup fitted. for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. a calorimeter is a device used to determine heat flow during a chemical or physical change. Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To remind you, endothermic reactions gain energy from the. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). calorimetry is the science of measuring heat flow. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Purpose Of Calorimeter Lab a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to determine heat flow during a chemical or physical change. To remind you, endothermic reactions gain energy from the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. calorimetry is the science of measuring heat flow. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). A doubled styrofoam cup fitted. Heat is defined as thermal energy flowing from an object at a higher temperature to one.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Purpose Of Calorimeter Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A doubled styrofoam cup fitted. To remind you, endothermic reactions gain energy from the. for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. a calorimeter is a device used to determine heat flow during. Purpose Of Calorimeter Lab.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Purpose Of Calorimeter Lab for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. A doubled styrofoam cup fitted. calorimetry is the science of measuring heat flow. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). Heat is defined as thermal energy flowing from an object at a higher temperature to. Purpose Of Calorimeter Lab.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Purpose Of Calorimeter Lab calorimetry is the science of measuring heat flow. To remind you, endothermic reactions gain energy from the. a calorimeter is a device used to determine heat flow during a chemical or physical change. for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Heat is defined as thermal energy flowing from an object at. Purpose Of Calorimeter Lab.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Purpose Of Calorimeter Lab a calorimeter is a device used to determine heat flow during a chemical or physical change. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). calorimetry is the science of measuring heat flow. a calorimeter is a device used for calorimetry, or the process of measuring the heat of. Purpose Of Calorimeter Lab.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Purpose Of Calorimeter Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. To remind you, endothermic reactions gain energy from the. a calorimeter is a device used for calorimetry, or the process of measuring. Purpose Of Calorimeter Lab.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Purpose Of Calorimeter Lab for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. A doubled styrofoam cup fitted. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter. Purpose Of Calorimeter Lab.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Purpose Of Calorimeter Lab a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. A doubled styrofoam cup fitted. for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. To remind you, endothermic reactions gain energy from the. Heat is defined as thermal energy flowing from an object at. Purpose Of Calorimeter Lab.
From www.thoughtco.com
Calorimeter Definition in Chemistry Purpose Of Calorimeter Lab a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To remind you, endothermic reactions gain energy from the. Heat is defined as thermal energy flowing from an object at a higher temperature to one. calorimetry is the science of measuring heat flow. a system is the specific. Purpose Of Calorimeter Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Purpose Of Calorimeter Lab a system is the specific place where a reaction or process is occurring (eg inside calorimeter). Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a calorimeter is a device. Purpose Of Calorimeter Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Purpose Of Calorimeter Lab Heat is defined as thermal energy flowing from an object at a higher temperature to one. To remind you, endothermic reactions gain energy from the. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical. Purpose Of Calorimeter Lab.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Purpose Of Calorimeter Lab To remind you, endothermic reactions gain energy from the. a calorimeter is a device used to determine heat flow during a chemical or physical change. A doubled styrofoam cup fitted. calorimetry is the science of measuring heat flow. for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. Heat is defined as thermal energy. Purpose Of Calorimeter Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Purpose Of Calorimeter Lab a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device. Purpose Of Calorimeter Lab.
From studylib.net
Calorimeter Lab Purpose Of Calorimeter Lab A doubled styrofoam cup fitted. Heat is defined as thermal energy flowing from an object at a higher temperature to one. a calorimeter is a device used to determine heat flow during a chemical or physical change. calorimetry is the science of measuring heat flow. a calorimeter is a device used for calorimetry, or the process of. Purpose Of Calorimeter Lab.
From kaffee.50webs.com
Lab Calorimetry Purpose Of Calorimeter Lab a system is the specific place where a reaction or process is occurring (eg inside calorimeter). for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. A doubled styrofoam cup fitted. a calorimeter. Purpose Of Calorimeter Lab.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Purpose Of Calorimeter Lab To remind you, endothermic reactions gain energy from the. a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. A doubled styrofoam cup fitted. a calorimeter is a device used to determine heat flow during a chemical or physical change. a system is the specific place where. Purpose Of Calorimeter Lab.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Purpose Of Calorimeter Lab calorimetry is the science of measuring heat flow. a system is the specific place where a reaction or process is occurring (eg inside calorimeter). for chemical reactions, we can use calorimetry to determine the enthalpy of reaction. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter. Purpose Of Calorimeter Lab.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Purpose Of Calorimeter Lab a system is the specific place where a reaction or process is occurring (eg inside calorimeter). a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. for chemical reactions, we can. Purpose Of Calorimeter Lab.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Purpose Of Calorimeter Lab a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat is defined as thermal energy flowing from an object at a higher temperature to one. A doubled styrofoam cup fitted. for. Purpose Of Calorimeter Lab.